DNP Announces that All Four DNP Medi-Ca(TM) Series Products Have Now Acquired AOAC PTM Certification
Dai Nippon Printing Co., Ltd. (DNP) is pleased to announce the Staphylococcus aureus count (SA) component of the DNP developed Medi-Ca(TM) microbial inspection film culture media series, has acquired Performance Test Method (PTM) certification from AOAC, the US based global authority on method validation and laboratory quality assurance.
The Medi-Ca series is a film-based product used in microbial culture media to improve the efficiency of quality control of microbial inspections carried out by food and beverage makers. In this latest development, the acquisition of PTM certification by SA means that all four components of the DNP Medi-Ca series, namely, the aerobic viable count (AC), the coliform colony count (CC), and the E.coli and coliform colony count (EC) have all acquired such certification.
With currently available counting methods using agar-based media, it has been necessary to involve the participation of skilled staff in the pre-treatment of the media. With Medi-Ca, however, the gelling agent and nutritional ingredient are coated on a sheet-form film, precluding the need for such pre-treatment and making handling easier, while also removing the need for the presence of such skilled staff and achieving improved testing operational efficiency, as a result.
[AOAC PTM Certification]
The international certification organization AOAC is an organization established with the objective of validating and certifying inspection methods for new developments relating to food, pharmaceuticals, feed and fertilizer, and cosmetics in the US. AOAC PTM certification is a testing method whose validity is confirmed by a third-party organization recognized by the AOAC, a recognition given only to those inspection kits that pass the rigorous inspection standards based on the AOAC philosophy.
[DNP's Medi-Ca Series]
- Simple & Easy
As the media can be used immediately after removing from the outer packaging, it can accommodate rapidly to emergency inspections. By dripping a diluted sample solution of the foods or beverages to be inspected into the center of a growth area and simply closing the cover film, it is possible to spread the said-solution throughout the growth area, making it possible to execute the process in an efficient manner.
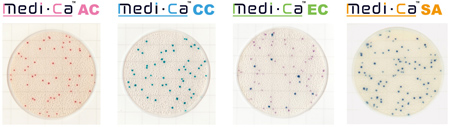
- Easy Interpretation - Brightly Colored Colonies
Medi-Ca produces brightly colored colonies distinguishable from food particles, making colony counting easier, and reducing variation in counting results. Medi-Ca AC has the additional benefi¬t of reducing the spreading of colonies of Bacillus spp. than the standard plate count method.
- Saving Space for Incubation & Reducing Amount of Waste
Since the volume of Medi-Ca is reduced to less than 1 / 20th of that of Petri dishes used in conventional tests, it reduces required spaces for storage and cultivation, and reduces the amount of waste after use. In addition, the simple work of dripping a solution and closing the cover film can be carried out while piling up Medi-Ca one after another, making it possible to save space in the work area.

[At left] Petri dish / [At right] Medi-Ca
[Medi-Ca Product Lineup and Pre-tax Pricing]
Aerobic viable count (AC) | 75,000 yen (per 1,000 sheet pack) |
Coliform colony count (CC) | |
E.coli and coliform colony grading (EC) | 47,500 yen (per 500 sheet pack) |
Staphylococcus aureus count (SA) | 75,000 yen (per 500 sheet pack) |
[Looking Ahead]
DNP will market the four Medi-Ca products mainly to food, beverage, snack manufacturers and restaurant chains, aiming for sales of 300 million yen in FY2021.
* Product prices, specification and service contents mentioned in this news release are current as of the date of publication. They may be changed at any time without notice.
- Select location
-
- Category
-